Michael Belkin’s Congressional Testimony on Hepatitis B Vaccine
October 3, 2012
by admin in Vaccines, Videos

This is Michael Belkin’s testimony before the House Reform Committee. He speaks authoritatively, as a statistician, on VAERS and the lunacy of the Hepatitis B vaccine in infants. He starts by saying:
My daughter Lyla Louise Belkin died on September 16th 1998 at the age of five weeks about 15 hours after receiving her second Hepatitis B vaccine booster shot. Lyla was a lively alert five-week-old baby when I last held her in my arms. Little did I imagine as she gazed intently into my eyes with all the innocence and wonder of a newborn child that she would die that night.
A transcript of Michael’s testimony is below the video.
Transcript of Michael’s Testimony
My daughter Lyla Rose Belkin died on September 16, 1998 at the age of five weeks, about 15 hours after receiving her second Hepatitis B vaccine booster shot. Lyla was a lively, alert five-week-old baby when I last held her in my arms. Little did I imagine as she gazed intently into my eyes with all the innocence and wonder of a newborn child that she would die that night. She was never ill before receiving the Hepatitis B shot that afternoon. At her final feeding that night, she was extremely agitated, noisy and feisty — and then she fell asleep suddenly and stopped breathing. The autopsy ruled out choking, The NY Medical Examiner ruled her death Sudden Infant Death Syndrome (SIDS).
But the NY Medical Examiner (Dr. Persechino) neglected to mention Lyta’s swollen brain or the hepatitis B vaccine in the autopsy report. The coroner spoke to my wife and I and our pediatrician (Dr. Zullo) the day of the autopsy and clearly stated that her brain was swollen. The pediatrician Dr. Zullo’s notes of that conversation are “brain swollen … not sure cause yet … could not see how recombinant vaccine could cause problem.”
SIDS is a diagnosis of exclusion .. “it wasn’t this, it wasn’t that, everything has been ruled out and we don’t know what it was.” A swollen brain is not SIDS. Through conversations with other experienced pathologists, I subsequently discovered that brain inflammation is a classic adverse reaction to vaccination (with any vaccine) in the medical literature.
I set out to do an investigation of the hepatitis B vaccine and attended a workshop at the National Academy of Sciences, Institute of Medicine on “Neo-Natal Death and the Hepatitis B Vaccine,” the Advisory Committee on Immunization Practices (ACIP) February’ meeting and a debate in New Hampshire between the Chairman of the ACIP Dr. Modlin and Dr. Waisbren about the safety of the hepatitis B vaccine. I also obtained the entire Vaccine Adverse Events Reporting System (VAERS) database on hepatitis B vaccine adverse reactions and have investigated it thoroughly.
These are my conclusions, supported by the following pages of text and analysis that are too lengthy to present in entirety in the time allotted for this appearance. Please read the results of my investigation, as it will help you understand the magnitude of the hepatitis B vaccine issue.
Newborn babies are not at risk of contracting the hepatitis B disease unless their mother is infected. Hepatitis B is primarily a disease of junkies, gays, and promiscuous heterosexuals
The vaccine is given to babies because health authorities couldn’t get those risk groups to take the vaccine
Adverse reactions out-number cases of the disease in government statistics
Nothing is being done to investigate those adverse reactions
Those adverse reactions include numerous deaths, convsions and arthritic conditions that occur within days after hepatitis B vaccination
The CDC is misrepresenting hypothetical, estimated disease statistics as real cases of the disease
The ACIP is recommending new vaccines for premature infants without having scientific studies proving it is safe
The US vaccine recommendation process is hopelessly compromised by conflicts of interest with vaccine manufacturers, the American Academy of Pediatrics and the CDC
Conclusion: If (as with the recently-recommended rotavirus vaccine) hepatitis B vaccine was recommended in 1991 without scientific proof that it was safe in a broad sample of racially and genetically diverse babies less than 48 hours old before they established that recommendation,then the CDC has been experimenting on babies like guinea pigs and this Committee should suspend that universal immunization policy.
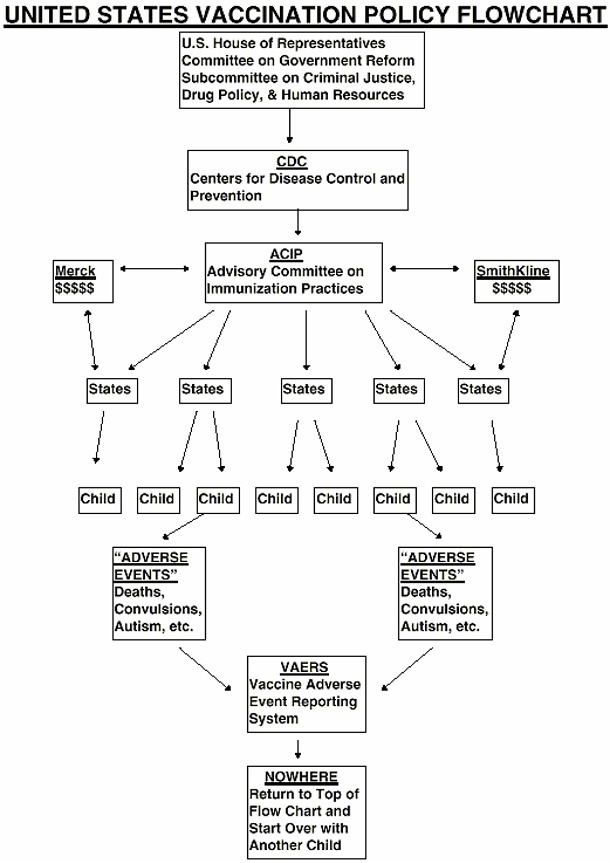
The hepatitis B vaccine was effectively mandated in 1991 for universal immunization of newborn babies by the Advisory Committee on Immunization Practices (ACIP) — an adjunct of the Centers for Disease Control and Prevention (CDC). Paradoxically, the CDC’s own Fact Sheet on the hepatitis B disease does not include newborn babies as a risk group for that disease. That Fact Sheet lists the risk groups as injection drug users, homosexual men, sexually active heterosexuals, infant/children of immigrants from disease-endemic areas, low socio-economic level, sexual/household contacts of infected persons, infants born to infected mothers, health care workers and hemodialysis patients NOT NEWBORN BABIES.
Question: Why then, did the ACIP establish a policy mandating that newborn babies not at risk of the disease be automatically administered the 3-shot hepatitis B vaccine as their first involuntary indoctrination into the pediatric care of America?
Answer: Here is that rationale from the original ACIP 1991 statement establishing the official vaccination policy “Hepatitis B Virus: A Comprehensive Strategy for Eliminating Transmission in the United States Through Universal Childhood Vaccination …” “In the United States, most infections occur among adults and adolescents … The recommended strategy for prevent/rig these infections has been the selective vaccination of persons with identified risk factors … However, this strategy has not lowered the incidence of hepatitis B, primarily because vaccinating persons engaged in high-risk behaviors, life-styles, or occupations before they become infected generally has not been feasible … Efforts to vaccinate persons in the major risk groups have had limited success. For example, programs directed at injecting drug users failed to motivate them to receive three doses of vaccine … In the United States it has become evident that HBV transmission cannot be prevented through vaccinating only the groups at high risk of infection … In the long term, universal infant vaccination would eliminate the need for vaccinating adolescents and high-risk adults … Hepatitis B vaccination is recommended for all infants, regardless of the HBsAg status of the mother … The first dose can be administered during the newborn period, preferably before the infant is discharged from the hospital, but no later than when the infant is 2 months of aqe …” (emphasis added).
So in the CDC and ACIP’s own words, almost every newborn US baby is now greeted on its entry into the world by a vaccine injection against a sexually transmitted disease for which the baby is not at risk ‘-because they couldn’t get the junkies, prostitutes, homosexuals and promiscuous heterosexuals to take the vaccine. That is the essence of the hepatitis B universal vaccination program.
Question: What are the risks and benefits for administering this vaccine to infants?
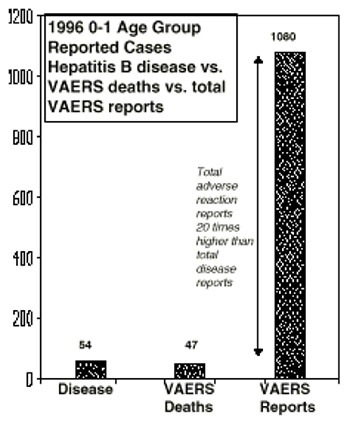
Answer: Hepatitis B is a rare, mainly blood-transmitted disease. In 1996 only 54 cases of the disease were reported to the CDC in the 0-1 age group. There were 3.9 million births that year, so the observed incidence of hepatitis B in the 0-1 age group was just 0.001%. In the Vaccine Adverse Event Reporting System (VAERS), there were 1,080 total reports of adverse reactions from hepatitis B vaccine in 1996 in the 0-1 age group, with 47 deaths reported. Total VAERS hepatitis B reports for the 0-1 age group outnumber reported cases of the disease 20 to 1.
Question: Why don’t they just screen the mother to see if she is infected with hepatitis B (since that is about the only way a baby is likely to get the disease), instead of vaccinating infants?
Answer: Selling vaccines is extremely profitable and the process of mandating vaccines is fraught with conflicts of interest between vaccine manufacturers, the ACIP and the American Academy of Pediatrics. The business model of having the government mandate everyone must buy your product is a monopolist’s delight.
Question: What studies are being done on the data from the FDA’s Vaccine Adverse Event Reporting System (VAERS)?
Answer: Absolutely nothing. The 25,000 reports are going into a drawer and being forgotten. How many reports are enough to show a drug or vaccine is dangerous — 2,500? 25,000? 250,000? Chen of the CDC and Ellenberg of the FDA monitor this data, write reports and deliver speeches about how VAERS hepatitis B adverse reaction reports show nothing out of the ordinary and show “the relative safety of HB vaccine when given to neonates and infants.” VAERS shows nothing of the kind. TAKE A LOOK AT THE VAERS DATA YOURSELF. The health authorities continue to negligently downplay the steady stream of serious adverse reactions to this vaccine and more infants and adults continue to die and suffer central nervous system and liver damage after HB vaccination.
Question: Why do the CDC, ACIP and Merck say that there are 140,000-320,000 new infections/yr (70,000-160,000 symptomatic infections/yr) when their own CDC data shows only 1O,000 reported cases year?
Answer: They are passing off estimated, hypothetical numbers as actual cases. This is statistical fraud. In the financial world such misrepresentation would lead to criminal charges. If a company inflated its earnings or revenues by 300% (as the CDC does hepatitis B disease statistics) and foisted those figures off as official data (and not some back-of-the-envelope guess-timate) – that company would be investigated by the SEC and sued by shareholders. Why doesn’t that happen in the medical world? There’s no regulator to keep the CDC honest. They do not say those figures are hypothetical estimates, they misrepresent the data. Go try to audit those 320,000 supposed new infections/yr. You will not find them. The whole exercise is designed to increase public hysteria about the risk of a low-risk disease so the CDC can extend it’s pervasive influence and Merck can increase its $900 million/year vaccine revenues.
Question: What process does the Center for Disease Control employ to make a vaccine recommendation?
I attended the February Advisory Committee on Immunization Practices (ACIP) meeting in Atlanta and was absolutely appalled. Every vote by the Committee on new vaccine mandates was unanimous (except for one dissenting vote on Rotavirus vaccine for premature infants). There was hardly any discussion of adverse reactions, the ACIP simply rubber-stamped every proposal on the agenda. I call it Vaccination Without Representation. In one instance, the ACIP passed a recommendation for Rotavirus vaccine for premature infants even though no scientific studies had been done showing it was medically safe. Dr. Modlin, (Chairman of the ACIP), said in a pro-hepatitis B vaccine debate in New Hampshire “How do we determine whether something is scientifically valid or not?… 1) Is the theory biologically plausible? 2) Has it been tested by appropriate methods? 3)Is the study well concluded? 4) Are the results statistically sound? But at the February ACIP meeting, when it came time for the ACIP to rubber-stamp approval of Rotavirus vaccine for premature infants, here are Modlin’s quotes from the official transcript: “.. available data are insufficient to fully establish the safety and efficacy of rotavirus vaccine in premature infants … there is a section under Adverse Events that details what little information there actually are with respect to premature infants … To my knowledge we don’t have data from a clinical trial specifically … Some bit of information from Seattle, as I recall, that had suggested that was a slight increase in relative risk for hospitalization for premature infants …Obviously a situation where we have to make a judgment in the absence of data, and with a vaccine that has not yet been tested in the group …” (ACIP transcript, pages 102-112) Modlin then held a vote and the recommendation for premature infants passed nine to one — Modlin voted yes, Dr. Glode against. This is a clear example of how the medical bureaucracy (led by the CDC and ACIP), is recommending vaccines without scientific evidence that those vaccines are safe in a broad sample of racially and genetically diverse infants.
What Should Be Done? This Committee should investigate the 1991 ACIP recommendation establishing universal hepatitis B vaccination of newborn babies in the hospital — and if (as with the Rotavirus vaccine example above) no studies were done to prove this was safe in a broad sample of racially and genetically diverse babies less than 48 hours old before they established that recommendation, then the CDC has been experimenting on babies like guinea pigs and this Committee should suspend that universal immunization policy.
VAERS ANALYSIS (Vaccine Adverse Event Reporting System)
I studied statistics at the University Of California at Berkeley and went on to develop sophisticated proprietary risk/reward statistical models at Salomon Brothers from 1986-91 — and in my subsequent, ongoing business provide statistical economic and financial forecasts to mutual funds, investment banks, pension funds and hedge funds.
I studied VAERS hepatitis B vaccine data obtained by the National Vaccine Information Center (NVIC) under the Freedom of Information Act. The data has some flaws (incomplete fields, some multiple reports) but any qualified, impartial quantitative analyst or statistician not affiliated with Merck, Smithkline, the CDC, the FDA or the AAP who examines these reports will find a clear and undeniable pattern of central nervous system (CNS) and liver disease striking thousands of people within 0-4 days after vaccination with hepatitis B vaccine any qualified, impadial quantitative analyst or statistician not affiliated with Merck, Smithkline, the CDC, the FDA or the AAP who examines these reports will find a clear and undeniable pattern of central nervous system (CNS) and liver disease striking thousands of people within 0-4 days after vaccination with hepatitis B vaccine. These reports have been ignored, explained away, or considered “acceptable” by the FDA, CDC and drug companies. This Committee should launch an investigation of the VAERS hepatitis B data by a team of independent scientists not beholden to vaccine manufacturers or the FDA/CDC bureaucracy. The following is intended to be a starting point for such an investigation. This does not profess to be a complete, exhaustive analysis — simply an overview, highlighting aspects of the data that may not previously have been brought to your attention.
The total 24,775 VAERS hepatitis B reports from July 1990 to October 31, 1998 show 439 deaths and 9673 serious reactions involving emergency room visits, hospitalization, disablement or death. Therefore, more than one third of total reports were serious events. 17,497 of those total reports were for hepatitis B vaccine only, the remainder were vaccine cocktails where hepatitis B was administered along with DPT, HIB, IPV, OPV, etc.
The hepatitis-B-vaccine-only reports show a shocking cluster of reactions in females starting in their teenage years (the male/female reporting ratio is balanced before age 16). For ages 16-55, 77% of VAERS reports are women — more than three times as many women as men are reporting adverse reactions to hepatitis B vaccine. The median onset of adverse event after vaccination is one day, 70% of reactions happen within four days of vaccination. independent scientists should investigate why females are more disposed to have adverse reactions to hepatitis B vaccine and/or report them to VAERS. One possible explanation is that nurses have to take this vaccine for their jobs and are thus more exposed than most adults to hepatitis B vaccine adverse reactions. Rather than dismiss that factor as an “over-reporting bias” as Dr. Chen of the CDC did at the February ACIP meeting, perhaps investigators might consider that nurses are alert health care workers and ought to be listened to with regard to the dangers of adverse events with any vaccine (rather than ignored). Personal case studies reported to the author have showed many teenage girls getting severe, debilitating adverse reactions to hepatitis B vaccine, having nothing to do with nursing. Do women have a greater vulnerability to auto-immune reactions to hepatitis B vaccine? Is the government discriminating against women by administering this vaccine without regard for genetic risk of CNS and liver disease? Those are questions that independent scientists should investigate.
A second area of concern is the VAERS reports involving hepatitis B vaccine administered with other vaccines (vaccine cocktails). Health officials are fond of dismissing those repeals as being attributable to hepatitis B vaccine, because of the multiple other antigens present (almost as if they wanted to cloak hepatitis B vaccine reactions from scrutiny). Let’s avoid that controversy and focus on the extremely disturbing VAERS data of hepatitis B vaccine with other vaccines. These reports amount to only one third of total reports (7,275), but account for two thirds of total deaths (291). The median onset of those deaths was 2 days after vaccination — displaying a clear temporal association. The median age of death was 0.5 years in this group. 50% of all hepatitis-B-vaccine-cocktail reports were serious (died, emergency room, hospitalized, disabled). I grouped convulsive reactions together from the hep-B-vaccine-cocktail data and found a deeply disturbing pattern. These were anything labeled convulsions, seizures or tremors in the VAERS hep-B-cocktail data. Of the 1189 such reports, fully 80% (950) were serious (died, ER, hospitalized, disabled) median age 0.5 years, median onset after vaccination 0 days (less than one day). Someone should do follow-up and find out what happened to those poor infants who suffered severe convulsions after a hepatitis B-multi-vaccine cocktail. In the personal reports I’ve taken of similar adverse reactions, the children were left brain damaged and developmentally disabled. Looking beyond the debate over whether VAERS reports of vaccine cocktails can be attributed to hepatitis B, the data strongly suggests combining multiple vaccines may be convenient and profitable for pediatricians — but fatal or debilitating for infants. Where are the scientific studies showing hepatitis B vaccine is safe to administer with DPT, HIB, IPV, OPV, etc.? Did anyone doing cost/benefit analysis for those studies include data showing the higher mortality and serious reactions present in the VAERS data? Why not? Is there an identifiable genetic marker in those who suffered convulsive reactions to screen out those vulnerable in the future? These are all matters for independent scientists to audit.
Another area that leaps out of the VAERS database is something I dubbed arthritic reactions. These are joint pains, tingling, numbness, aching, fatigue, etc. I found 2,400 of those reports in just a quick survey of the first reporting column of VAERS (hepatitis B vaccine only). Almost one half of those are serious, involving an ER visit, hospitalization, death or disablement. These are the type of adverse reactions reported by many adults who are forced to take the hepatitis B vaccine for their jobs. In the reports of such adverse reactions I’ve taken, the symptoms do not go away, most patients complain it gets worse over time. Scientists not corrupted by drug company or CDC/FDA institutional bias should examine the thousands of VAERS hepatitis B arthritic reaction reports and develop a diagnosis of their hepatitis B vaccine-related illness.
Anyone who doubts if hepatitis B vaccine adverse reactions exist should sit down and read the symptoms and text comments of a random selection of VAERS reports. When one does so, they will find a similar but wide-ranging list of CNS and liver reactions that occur within days of vaccination. The Merck package insert claims “Injection site reactions and systemic complaints were reported following 17% and 15% on the injections, respectively.” The standard rule of thumb is only about 10% of reactions are reported to VAERS. So the actual number and full horror of the hepatitis B vaccine reaction story is potentially much larger than even VAERS suggests.
[Note: Emphases added by Michael Belkin.]