January 17, 2013
Antipsychotics are notoriously dangerous drugs, causing life-threatening diabetes, along with severe neurological damage. Authors of a new study claim that Saphris is harmless, but examination shows that their study doesn’t come close to showing safety. It’s pure junk science.

Image derived from Catarina Carneiro de Sousa’s far superior work.
Creative Commons Share and Share Alike License.
by Heidi Stevenson
Antipsychotic pharmaceutical drugs, such as Zyprexa, are noted for their severe adverse effects. The second generation of these drugs, such as Zyprexa (olanzapine) and Abilify (aripiprazole) were initially touted as relatively free of these effects, but the truth came out fairly quickly: They are at least as bad as their predecessors, possibly even worse. So now, there’s an attempt to find a second generation antipsychotic that doesn’t carry the same extreme dangers.
In 2009, the FDA approved two new second-generation antipsychotics into psychiatry’s repertoire: asenapine (Saphris) and iloperidone (Fanapt). Now—well over two years later—we’re finally seeing a study on rats comparing the health effects of these two new drugs with olanzapine (Zyprexa). This sort of study should, of course, have been done before these drugs were even tested on humans, not after they’ve been approved! But, the story gets even worse.
In this case, the comparison was made with olanzapine (Zyprexa), which is infamous for causing severe metabolic syndrome leading to diabetes. This study, just published in PLoS, purports to show that one of them, asenapine – Saphris – not only fares well compared to olanzapine, but also does no harm related to metabolic syndrome.
The problem is that nothing in the study actually supports the claim.
The Study
It should come as no surprise, since the authors are neck-deep in Big Pharma’s largesse. There are consulting and lecture fees from AstraZeneca, Bristol-Myers Squibb, Janssen, Sunovion, Pfizer, and Otsuka. Paid advisory roles from Novartis, Roche Canada, Eli Lilly Canada, and the Canadian Agency for Drugs and Technology in Health. Royalties for antibody licenses from the University of British Columbia. And an educational grant from BMS Canada.
They used female rats, injected them with antipsychotic drugs, and tested for metabolic problems, including the intraperitoneal glucose tolerance test (IGTT).
The rats were given baseline IGTT exams before drugs were administered.
Dosage
First, we need to know the standard dosing of each of these drugs on patients:
- Asenapine (Saphris) is given in 5 mg. doses, up to 20 mg. per day.
- Iloperidone (Fanapt) is given in 6-12 mg. doses, up to 24 mg. per day.
- Olanzapine (Zyprexa) is given in 5-10 mg doses, up to 20 mg. per day.
Dosing of each of these drugs is similar. However, the study’s authors didn’t seem to take that into consideration, nor did they note that there’s a cumulative effect over time in causing metabolic disorder.
The experimental rats were all injected subcutaneously with single doses of either placebo or varying levels of each drug. They were then put through glucose tests to see the metabolic effects of the drug or placebo.
- Rats were first given asenapine in doses of 0.01, 0.05, 0.1, 0.5, or 1 mg/kg. or injected with a placebo.
- A week later, the same rats were injected with iloperidone in doses of 0.03, 0.5, 1.0, 5.0, or 10.0 mg/kg., or injected with placebo.
- A week after the iloperidone dosing, the same rats were injected with olanzapine in doses of 0.1, 0.5, 1.5, 5.0, or 10.0 mg/kg—or 0.1, 0.5, 1.5, 5.0, or 15.0 mg/kg, depending of whether you refer to the written text of the report or the associated graph. (This discrepancy alone should give pause for whether the study holds any validity. It most assuredly indicates that any peer reviews were sorely lacking!)
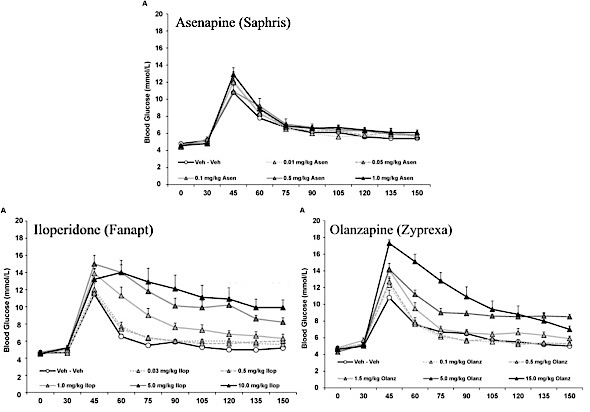
The results given by the study seem quite impressive and appear to virtually absolve asenapine of causing any long term metabolic harm. Here are the study’s graphic results:
Notice that it shows a glucose spike right after administration of glucose at about 45 minutes in all the rats. In those given asenapine, the spike wasn’t as high and reduced more quickly than for either iloperidone or olanzapine. Both iloperidone’s and olanzapine’s glucose levels were apparently far worse. However, a closer look shows that glucose levels of rats given any of the drugs at the same levels reduced by similar amounts over time.
Let’s consider there highly significant points:
- The iloperidone doses were 3 times greater than asenapine at the low end and 10 times greater at the high end.
- The olanzapine doses were 10 times greater than asenapine at the low end and 10 or 15 times greater at the high end, depending on whether you refer to the study’s text or graphs..
- The rats were not divided into groups for testing each drug. Instead, all the rats were given all the drugs in the order of asenapine first, then iloperidone, and olanzapine last.
No consideration was given for the possibility that the earlier dose of one antipsychotic might affect the results of those given later. This alone is a fatal flaw that makes this study useless.
Worse, though, are the incredibly large differences in dosing. It cannot be justified by real-life dosing of patients, since those are approximately the same.
Comparing different doses is like comparing apples and oranges, unless those doses are equivalent to real-world dosing. That, though, is clearly not the case here. The results for higher dosing levels of iloperidone and olanzapine serve as nothing but a distraction.
Study’s Claim
The study concluded:
In preclinical models, asenapine shows negligible metabolic liability. By contrast, iloperidone exhibits substantial metabolic liability, comparable to olanzapine.
They also stated:
These overall findings reconfirm the powerful effect of the SGA [second generation antipsychotic] olanzapine in animal models of glucose control and uptake, and demonstrate for the first time that similar-to-greater magnitude effects were observed with iloperidone, while asenapine shows minimal metabolic liability.
The authors gave rats doses of Zyprexa and Fanapt that are at least ten times greater than Saphris, and then compared the results of those higher doses with Saphris. That Saphris showed less harm at the lower doses is translated into the claim that Saphris “shows negligible metabolic liability”. This is pure nonsense!
Comparing Saphris’ results when given at the same levels at Fanapt and Zyprexa shows that they’re actually quite similar, a fact that the authors chose to ignore.
Junk Science
This could serve as the definition of junk science. It makes a comparison that’s no better than comparing blond wigs in China with black boots in England. Comparing dramatically different doses of drugs tells us virtually nothing. The researchers’ claim that Saphris “shows minimal metabolic liability” is simply not shown.
Why should we trust anything that’s published in medical journals when this sort of thing can be published in a journal that’s supposedly respected?
Sources:
- Metabolic Side-Effects of the Novel Second-Generation Antipsychotic Drugs Asenapine and Iloperidone: A Comparison with Olanzapine, PLoS;, Heidi N. Boyda, Ric M. Procyshyn, Catherine C. Y. Pang, Erin Hawkes, Daniel Wong, Chen Helen Jin, William G. Honer, Alasdair M. Barr; doi:10.1371/journal.pone.0053459






Leave a comment