Japan's Health Ministry Withdraws Cervical Cancer Vaccine Recommendation
June 17, 2013
by DAVE MIHALOVIC
Japan's health ministry issued a nationwide notice that cervical cancer vaccinations should no longer be recommended for girls due to several hundred adverse reactions to the vaccines reported.
A publication in the Annals of Medicine has exposed the fraudulent nature of Human papillomavirus (HPV) vaccines such as Gardasil and Cervarix. Key messages the researchers reported include a lack of evidence for any HPV vaccines in preventing cervical cancer and lack of evaluation of health risks.
One of the problems with vaccinations such as HPV is that they are not preventative, they do compromise safety and physicians will never provide accurate explanations of vaccine risks and benefits because they do not know themselves. Physicians can only rely on the information from vaccine manufacturers and since long-term pharmacokinetic effects which study the bodily absorption, distribution, metabolism and excretion of vaccines and their ingredients are never examined or analyzed, a Physician can never fully inform of patient of any benefits or risks.
“It is necessary to gather information immediately to accurately grasp how often (the side effects) are occurring,” said Mariko Momoi, who chairs the panel at the Health, Labor and Welfare Ministry that decided to suspend the recommendation. Momoi is vice president of the International University of Health and Welfare.
Cervical cancer vaccines are a recent addition to the regular vaccination list and were added after a revision to the Preventive Vaccination Law took effect in April. The government’s subsidy program for vaccination against cervical cancer started in 2010.
The two vaccines sold in Japan are Cervarix, made by GlaxoSmithKlein PLC of Britain, and Gardasil, made by Merck Sharp & Dohme, known as Merck & Co. in the United States.
Mika Matsufuji, 46, who represents an association of cervical cancer vaccination victims’ parents, said the health panel’s decision was a “big step forward.” Her daughter, who was vaccinated with Cervarix in 2011, lost the ability to walk and is now in a wheelchair, she said.
The group is calling for the vaccinations to be halted.
Those subject to the vaccination range from six-graders of elementary schools to first-year students of senior high schools.
“We welcome the decision not to recommend the vaccination even though it is a small step,” said Mika Matsufuji, head of a group of parents who say their children have suffered side effects from the vaccination. “Parents can decide whether their children should receive the vaccination or not.”
At present there are no significant data showing that either Gardasil or Cervarix (GlaxoSmithKline) can prevent any type of cervical cancer since the testing period employed was too short to evaluate long-term benefits of HPV vaccination. The longest follow-up data from phase II trials for Gardasil and Cervarix are 5 and 8.4 years, respectively, while invasive cervical cancer takes up to 20 -40 years to develop from the time of acquisition of HPV infection.
Persistent HPV infections caused by high-risk HPVs will usually not lead to immediate precursor lesions, let alone in the longer term to cervical cancer. The reason for this is that as much as 90% HPV infections resolve spontaneously within 2 years and, of those that do not resolve, only a small proportion may progress to cancer over the subsequent 20 - 40 years. Moreover, research data show that even higher degrees of atypia can either resolve or stabilize over time. Thus, in the absence of long-term follow-up data, it is impossible to know whether HPV vaccines can indeed prevent some cervical cancers or merely postpone them.
- To date, the efficacy of HPV vaccines in preventing cervical cancer has not been demonstrated, while vaccine risks remain to be fully evaluated.
- Current worldwide HPV immunization practices with either of the two HPV vaccines appear to be neither justified by long-term health benefits nor economically viable, nor is there any evidence that HPV vaccination (even if proven effective against cervical cancer) would
reduce the rate of cervical cancer beyond what Pap screening has already achieved.
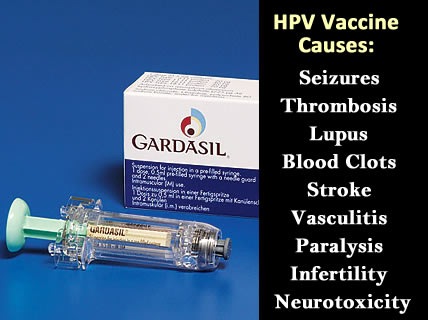
- Cumulatively, the list of serious adverse reactions related to HPV vaccination worldwide includes deaths, convulsions, paraesthesia, paralysis, Guillain-Barre syndrome (GBS), transverse myelitis, facial palsy, chronic fatigue syndrome, anaphylaxis, autoimmune disorders, deep vein thrombosis, pulmonary embolisms, and cervical cancers.
- Because the HPV vaccination programme has global coverage, the long-term health of many women may be at risk against still unknown vaccine benefits.
In the United States, thanks to the wealth of information available on the HPV vaccine fraud, the proportion of insured girls and young women completing the human papillomavirus (HPV) vaccine among those who initiated the series has dropped significantly -- as much as 63 percent -- since the vaccine was approved in 2006, according to new research from the University of Texas Medical Branch (UTMB) in Galveston.
Here are some examples of what Merck the manufacturer does not know about Gardasil and is consequently threatening the health of every person who is injected with this vaccine.
Summing up their evidence, the authors in the Annals of Medicine publication stated that the presentation of partial and non-factual information regarding cervical cancer risks and the usefulness of HPV vaccines, as cited above, is neither scientific nor ethical. None of these practices serve public health interests, nor are they likely to reduce the levels of cervical cancer. Independent evaluation of HPV vaccine safety is urgently needed and should be a priority for government sponsored research programmes. Any future vaccination policies should adhere more rigorously to evidence-based medicine as well as strictly follow ethical guidelines for informed consent.
Sources: