BEC CREW
28 MAY 2015
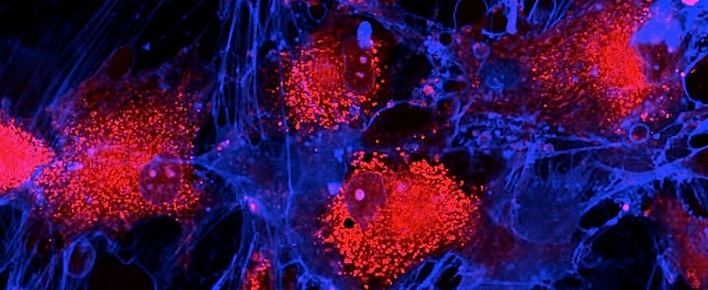
Image: Fibroblasts (skin cells) labeled with fluorescent dyes. Mitochondrias are in red, memrane is in blue.
Credit: Vshivkova/Shutterstock.com
They made a 97-year-old cell line behave as good as new.
By altering the behaviour of two genes responsible for the production of simple amino acids in human cells, scientists have gained a better understanding of how the process of ageing works, and how we could delay or perhaps even reverse it.
The team, led by Jun-Ichi Hayashi at the University of Tsukuba, targeted two genes that produce the amino acid glycine in the cell’s mitochondria, and figured out how to switch them on and off. By doing this, they could either accelerate the process of ageing within the cell, which caused signifiant defects to arise, or they could reverse the process of ageing, which restored the capacity for cellular respiration. Using this technique to produce more glycine in a 97-year-old cell line for 10 days, the researchers restored cellular respiration, effectively reversing the cell line’s age.
The finding brings into question the popular, but more recently controversial, mitochondrial theory of ageing, which puts forward the notion that an accumulation of mutations in mitochondrial DNA leads to age-related defects in the mitochondria - often referred to as the cell’s powerhouses because they are responsible for energy production and cellular respiration. Defects in the cell’s mitochondria lead to damage in the DNA, and an accumulation of DNA damage is linked to age-related hair loss, weight loss, spine curvature, osteoporosis, and a decreased lifespan.
But is this theory accurate? The results of Hayashi’s study support an alternative theory to ageing, which proposes that age-associated mitochondrial defects are caused not by the accumulation of mutations in mitochondrial DNA, but by certain crucial genes being turned on and off as we get older.
The team worked with human fibroblast cell lines gathered from young people - from foetus-age to 12 years old - and the elderly, from 80 to 97 years old. They compared the capacity for cellular respiration in the young and old cells, and found that while the capacity was indeed lower in the cells of the elderly, there was almost no difference in the amount of DNA damage between the two. This calls into question the mitochondrial theory of ageing, the team reports in the journal Scientific Reports , and suggests instead that the age-related effects they were seeing were being caused by a process known as epigenetic regulation.
Epigenetic regulation describes the process where the physical structure of DNA - not the DNA sequence - is altered by the addition or subtraction of chemical structures or proteins, which is regulated by the turning on and off of certain genes. "Unlike mutations that damage that sequence, as in the other, aforementioned theory of ageing, epigenetic changes could possibly be reversed by genetically reprogramming cells to an embryonic stem cell-like state, effectively turning back the clock on ageing," says Eric Mack at Gizmag.
Hayashi and his team supported this theory by showing that they could turn off the genes that regulate the production of glycine to achieve cellular ageing, or turn them on for the restoration of cellular respiration. This suggests, they say, that glycine treatment could effectively reverse the age-associated respiration defects present in their elderly human fibroblasts.
"Whether or not this process could be a potential fountain of youth for humans and not just human fibroblast cell lines still remains to be seen, with much more testing required," Mack points out at Gizmag. "However, if the theory holds, glycine supplements could one day become a powerful tool for life extension."
We’ll just have to wait and see. The faster we can solve the debate over how ageing actually works, the faster we can figure out how to delay it.






Leave a comment